New research simplifies the process of harvesting solar energy to generate fuels
04/13/2019 / By Edsel Cook
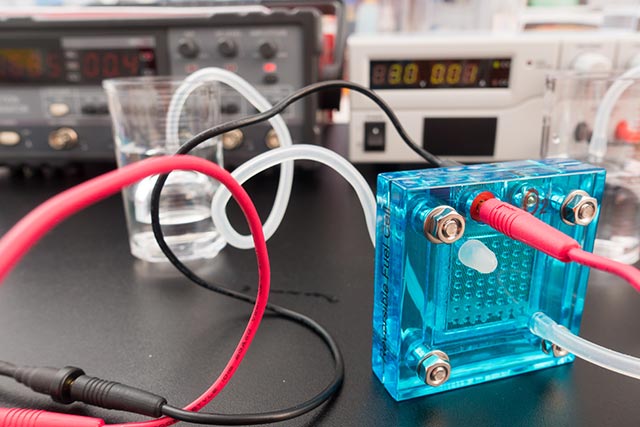
Harvesting hydrogen gas from water is going to be a much cheaper process thanks to the findings of New York-based researchers. They found a way to make the water splitting process much simpler, which could increase the overall efficiency of hydrogen fuel production and save on expended catalysts.
As its name implies, water splitting is a method where the water molecules are broken up into their component hydrogen and oxygen atoms. The hydrogen atoms are collected and burned as a clean fuel that generates a lot of energy without producing air pollutants.
The process is helped along by catalysts that increase the chances of the chemical reactions that split water into hydrogen and oxygen. These catalysts are triggered by an external source of energy. For example, photocatalysts are activated by light.
The Binghamton University, State University of New York (SUNY Binghampton) research team developed a way to improve the efficiency of the photocatalyst used in water splitting. They published their findings in the Journal of American Chemical Society. (Related: Engineers develop revolutionary technology that uses solar energy to turn seawater into potable water.)
A new way to increase the performance of photocatalysts for water splitting
“For water splitting, we use visible light to generate photo-excited negative electrons and positive holes that are then separated in order to catalyze water into oxygen and hydrogen gases,” said SUNY Binghampton researcher Louis Piper. “Storing gases is more straightforward (and cheaper) than employing battery set-ups, so this approach has the benefit of clean energy harvesting and storage.”
Piper and his colleagues wanted to increase the efficiency of the vanadium pentoxide (M-V205) used for splitting water. This particular photocatalyst is shaped like a wire and is measured in mere nanometers.
Their chosen method of improving the catalyst involved “doping.” They added metal ions into the nanostructure of vanadium pentoxide, which served as quantum dots that improved the electronic structure of the catalyst.
The researchers found that doping the photocatalyst increased the highest filled energy levels in the catalyst. The photo-excited electrons and positive holes produced by visible light would split apart faster.
“Using computation and chemical intuition, we predicted doping with Sn2+ ions would result in excellent energy alignment and efficient charge separation,” Piper explained. “We saw a ten-fold increase in the amount of solar-harvested hydrogen we obtained.”
Improved photocatalyst lasts longer and can be used more often
Another benefit to doping is that the holes moved from the quantum dots to the nanowires with greater efficiency. Not only did the more efficient movement increase the energy released by the process, it also extended the useful lifespan of the photocatalyst itself.
If large amounts of the holes accumulated on the quantum dots, the catalyst would undergo a parasitic reaction called photo-corrosion. The light would cause the structure to start deteriorating, making it less effective with each use until it finally broke down.
By reducing the amount of positive holes that remained on the quantum dots, the chances of photo-corrosion are decreased. The vanadium pentoxide photocatalyst will last longer and can be used multiple times.
Next up for Piper’s team is to improve the production of hydrogen gas during the water splitting process. They are thinking of adding platinum atoms to the quantum dots of the vanadium pentoxide photocatalyst.
The platinum is expected to serve as a catalytic site for the electrons. However, the noble metal is also very expensive, so the researchers are looking for cheaper alternatives.
Sources include:
Tagged Under: breakthrough, catalyst, Chemistry, discoveries, electricity, future science, hydrogen fuel, innovation, nanotech, photocatalyst, renewable energy, science and technology, solar energy, water splitting